How Accurate is the Georgia DUI Breath Test?
The Alcosensor Field Test
The Alcosensor, or handheld breath test, can be useful if you are sober or have consumed very little alcohol–two drinks or less. The numerical results of an Alcosensor Test are not admissible in court; the only admissible result from the test is whether the accused blew a positive or negative reading. The Alcosensor is highly inaccurate and has no ability to filter or distinguish between breath alcohol and other substances that mimic alcohol in infrared light. However, many police place undeserved weight on Alcosensor results in their arrest, despite the numeric result being inadmissible.

The State-Administered Breath Test
The Intoxilyzer 5000 is the State-administered breath test. This test is supposedly more accurate than the Alcosensor. It has filters for interferents like Acetone, mouth alcohol, radio frequency interference and other solvents that mimic alcohol on the infrared spectrum. The machine has a margin of error of +/- 0.02, which is 25% of the legal limit (0.08 grams). Because of this margin of error, the police must take two breath samples and the results must not vary more than 0.02 grams. Also, the police can only use the lowest of two breath samples. If an individual does not provide two breath samples, they can be charged with a “refusal” which carries a one year license suspension. However, if agreed upon, the police can only make you blow a maximum of four times.
What?! No Warranty?
Did you know that the company which manufactures the Intoxylizer 5000, CMI, Inc., will not warranty that the device is capable of measuring blood alcohol levels?
History, Science, and Limitations of Breath Testing
A breathalyzer is a device used for estimating blood alcohol content (BAC) from a breath sample. “Breathalyzer” is the brand name of a model made by one manufacturer (originally Smith and Wesson, later National Draeger), but has become a genericized trademark for all such instruments. Intoxilyzer, Intoximeter, Alcotest, Alcosensor and Datamaster are the other most common brand names in use today. The U.S. Government’s National Highway Traffic Safety Administration maintains a “Conforming Products List” of breath alcohol devices approved for law enforcement use.
Though technologies for detecting alcohol vary, it’s widely accepted that Dr. Robert Borkenstein (1912-2002), a captain with the Indiana State Police and later a professor at Indiana University, is regarded as the first to create a device that measures a subject’s alcohol level based on a breath sample. In 1954, Borkenstein invented his breathalyzer, which used chemical oxidation and photometry to determine alcohol concentration. The invention of the breathalyzer provided law enforcement with a non-invasive test that produced immediate results that could be used to determine an individual’s level of intoxication. The various versions of the Breathalyzer were made by Smith and Wesson, the gun manufacturer, until it was sold to the German company National Draeger.
Breath analyzers don’t directly measure blood alcohol content or concentration; this requires the analysis of a blood sample. Instead, they estimate BAC indirectly by measuring the amount of alcohol in one’s breath. Two technologies are most prevalent. Evidentiary machines, used by police forces, generally utilize infrared spectrophotometer technology. Hand-held field testing devices, less accurate but becoming increasingly popular with law enforcement, are based on electro-chemical fuel cell analysis and are used by officers in the field as a form of “field sobriety testing.” These handheld breath tests are commonly called PBT (preliminary breath test) or PAS (preliminary alcohol screening).
There are several models of breath-alcohol analyzers that are intended for the consumer market. These hand-held devices are less expensive and usually much smaller than the devices used by law enforcement. They are generally less accurate but can still give a useful indication of the user’s BAC. Almost all of these devices use less expensive tin-oxide semiconductor alcohol sensors, which are not as stable as fuel cell sensors or infrared devices and are more prone to false positives. Breath alcohol analyzers sold to consumers in the United States are required to be certified by the Food and Drug Administration, while those used by law enforcement must be approved by the Department of Transportation’s National Highway Traffic Safety Administration.
If the suspect refuses to submit to chemical testing, he will be charged with a violation of the DUI less safe law: driving with a BAC of .08 or lower (.02 in most states for people under 21), but still being “less safe” to drive. A DUI per se violation is when someone agrees to chemical testing and it is discovered that they were driving with a BAC of .08 or higher. The breathalyzer reading will be offered as evidence of that crime, although the issue is the BAC at the time of driving rather than at the time of the test. While BAC tests are not necessary to prove a defendant was under the influence, laws in most states require the jury to presume that he was under the influence if his BAC was over .08% when driving. You can, however, refute this presumption. The jury may also disregard the test if they find it unreliable or if other evidence establishes reasonable doubt. If a defendant refused to take a breathalyzer test, most states allow evidence of that fact to be introduced. In many states, the jury is instructed that they can draw a permissible inference of “consciousness of guilt.” In drunk driving cases in Massachusetts and Delaware, if the defendant refuses the breathalyzer there can be no mention of the test during the trial.
Some states don’t permit data or “readings” from hand-held PBTs to be presented as evidence in court. If they’re admissible at all, they can only be used to either show the presence of alcohol, or as a pass-fail field sobriety test to help an officer determine probable cause for arrest. South Dakota does not permit data from any type or size breath tester and relies entirely on blood tests to ensure accuracy.
Common Sources of Error:
Research indicates that breathalyzers are not as accurate and reliable as widely believed. Breath testers can be very sensitive to temperature, for example, and will give false readings if they aren’t adjusted or recalibrated to account for ambient or surrounding air temperatures. The temperature of the subject is also very important. Each one degree Celsius of body temperature above normal (+1.8 degrees Fahrenheit) will cause a substantial elevation (about 7%) in apparent BAC.
Breathing pattern can also significantly affect breath test results. One study found that the BAC readings of subjects decreased 11-14% after running up one flight of stairs and 22-25% after running up two flights. Another study found a 15% decrease in BAC readings after vigorous exercise or hyperventilation. Hyperventilation for 20 seconds has been shown to lower the reading by approximately 10%. On the other hand, holding your breath for 30 seconds can increase the breath test result by about 15%.
Some breath analysis machines assume a hematocrit (cell volume of blood) of 47%. However, hematocrit values range from 42-52% in men and from 37 to 47% in women. A person with a higher hematocrit will have a falsely high BAC reading. Hemocrit levels can throw off a breath test as much as 14%. See, Physiological Errors Associated with Alcohol Breath Testing, by Michael P. Hlastala, Ph.D. Professional bicycle racers monitored in Australia regularly recorded hemocrit levels at about 50%, and even as high as 52%, when they were verified as non-doping or not utilizing performance enhancing drugs such EPO. Further, studies of professional bicycle racers found a 6% reduction in plasma when blood was tested after 30 minutes of standing. Simply standing for 30 minutes can cause your hemocrit level to rise from 49% to 50.5%. BLOOD TESTING FOR PROFESSIONAL CYCLISTS: What’s a fair hematocrit limit? David T. Martin , Michael Ashenden, Robin Parisotto, David Pyne, Allan G. Hahn, Department of Physiology and Applied Nutrition, Australian Institute of Sport, Belconnen, Australia. Now consider that almost every person tested for breath alcohol spends 30 seconds standing to perform alcohol field sobriety evaluations. This could in turn trigger higher breath alcohol levels as a result of posture-induced increases in hemocrit.
Common sources of error arise from a law enforcement officer’s failure to use the devices properly, or an administrator’s failure to properly maintain or re-calibrate the devices as required.
Research indicates that breath tests can vary at least 15% from actual blood alcohol concentration. An estimated 23% of individuals tested will have a BAC reading higher than their true BAC. And police in Victoria, Australia use breathalyzers that give a recognized 20% tolerance on readings. Noel Ashby, Victoria Police Assistant Commissioner (Traffic & Transport) claims that this tolerance is to allow for different body types.
Call (404) 333-0706 or email today for a FREE CONSULTATION.
Non-specific Analysis:
One major problem with most breathalyzers is non-specificity: the machines not only identify the ethyl alcohol (or ethanol) found in alcohol beverages, but also other substances similar in molecular structure. Essentially, the machines project an infra-red beam of energy through the captured breath in the sample chamber. The more energy that is absorbed by compounds containing the methyl group in their molecular structures, the less reaches the detector on the other side — and the higher the reading. The assumption is that the compound containing the methyl group is probably ethyl alcohol. However, studies indicate that over one hundred compounds containing the methyl group have been identified on the human breath and will be incorrectly detected as ethyl alcohol. Importantly, the effect is cumulative: the more methyl group compounds absorbing the infrared energy, the higher the false breath test result.
The National Highway Traffic Safety Administration (NHTSA) has found that dieters and diabetics can have acetone levels hundreds and even thousands of times higher than those in others. This is due to non-specificity: acetone is one of the many substances that can be falsely identified as ethyl alcohol by some breath machines.
Substances in the environment can also lead to false BAC readings. For example, an alcohol-free subject was asked to apply a pint of contact cement to a piece of plywood and then to apply a gallon of oil-based paint to a wall. The total activity lasted about an hour. Twenty minutes later the subject was tested on an Intoxilyzer, which registered a BAC of .12. This level is 50% higher than a BAC of .08, which constitutes legal intoxication.
Any number of other products found in the environment can cause erroneous BAC results. These include compounds found in lacquers, paint removers, celluloid, gasoline, and cleaning fluids. Other common things that can cause false BAC levels are alcohol, blood or vomit in the subject’s mouth, electrical interference from cell phones and police radios, tobacco smoke, dirt, and moisture.
Partition Ratio:
Breathalyzers assume that the subject being tested has a 2100-to-1 partition ratio in converting alcohol measured in the breath to estimates of alcohol in the blood. This means that the machine is programmed to assume that the subject’s blood will have 2100 parts of ethyl alcohol in it for every part detected in the breath sample. However, this assumed “partition ratio” varies from 1300:1 to 3100:1 or wider among individuals or within a given individual over time. Assuming a true (and legal) blood-alcohol concentration of .07%, for example, a person with a partition ratio of 1500:1 would have a breath test reading of .10% — over the legal limit.
Mouth Alcohol:
One of the most common causes of inaccurately high breathalyzer readings is the existence of mouth alcohol. In analyzing a subject’s breath sample, the breathalyzer’s internal computer is making the assumption that the alcohol in the breath sample came from alveolar air — that is, air exhaled from deep within the lungs. However, alcohol may have come from the mouth, throat or stomach for a number of reasons. The most obvious is that the individual has recently consumed some alcohol; it usually takes 15-20 minutes for the alcohol to dissipate through the rinsing action of saliva.
The problem with mouth alcohol being analyzed by the breathalyzer is that it was not absorbed through the stomach and intestines and passed through the blood to the lungs. In other words, the machine’s computer is mistakenly applying the “partition ratio” (see above) and multiplying the result. Consequently, a very tiny amount of alcohol from the mouth, throat or stomach can have a significant impact on the breath alcohol reading.
Other than recent drinking, the most common source of mouth alcohol is from belching or burping, or in medical terms “eructation.” This causes the liquids and/or gases from the stomach — including any alcohol — to rise up into the soft tissue of the esophagus and oral cavity, where it will stay until it has dissipated. The American Medical Association concludes in its Manual for Chemical Tests for Intoxication (1959): “True reactions with alcohol in expired breath from sources other than the alveolar air (eructation, regurgitation, vomiting) will, of course, vitiate the breath alcohol results.” For this reason, police officers are supposed to keep a DUI suspect under observation for at least 15 minutes prior to administering a breath; in reality, however, many if not most officers, are unwilling to stand around watching a suspect for a quarter of an hour.
Acid reflux, or gastroesophageal reflux disease, can greatly exacerbate the mouth alcohol problem. The stomach is normally separated from the throat by a valve, but when this valve becomes herniated, there is nothing to stop the liquid contents in the stomach from rising and permeating the esophegus and mouth. The contents — including any alcohol — is then later breathed into the breathalyzer. See Kechagias, et al., “Reliability of Breath-Alcohol Analysis in Individuals with Gastroesophageal Reflux Disease”, 44(4) Journal of Forensic Sciences 814 (1999).
Mouth alcohol can also be created in other ways. Dentures, for example, will trap alcohol. Periodental disease can also create pockets in the gums which may contain alcohol for longer periods than normal. And recent use of mouthwash or breath freshener — possibly to disguise the smell of alcohol when being pulled over by police — contain fairly high levels of alcohol.
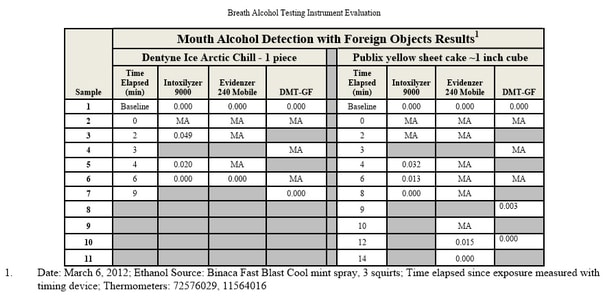
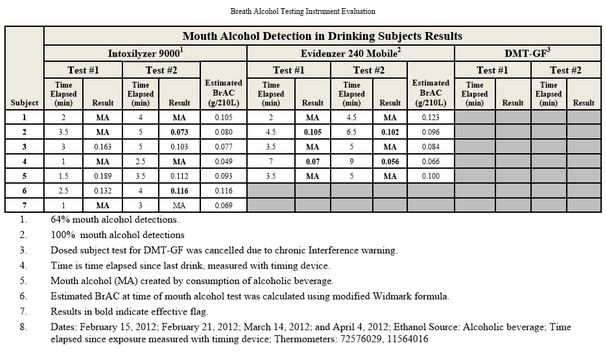
Testing During Absorptive Phase:
One of the most common sources of error in breath alcohol analysis is simply testing the subject too early — while his or her body is still absorbing the alcohol. Absorption of alcohol continues for anywhere from 45 minutes to two hours after drinking, sometimes longer. Peak absorption normally occurs within an hour but can range from as short as 15 minutes to as long as 2.5 hours.
During this absorptive phase, the distribution of alcohol throughout the body is not uniform. Uniformity of distribution — called equilibrium — will not occur until absorption is complete. In other words, some parts of the body will have a higher blood alcohol content (BAC) than others. One aspect of this non-uniformity is that the BAC in arterial blood will be higher than in venous blood (laws generally require blood samples to be venous). During peak absorption, arterial BAC can be as much as 60 percent higher than venous.
This standard is relevant to breath alcohol analysis because the alveolar sacs in the lungs are bathed by arterial blood, not venous. Because the alveolar sacs are bathed by arterial blood, the diffusion of alcohol through the sacs and into the lung air will reflect the BAC of the body’s arterial blood. Therefore the breath sample obtained by the machine will be reflective of pulmonary BAC — which, during absorption, will be higher than venous BAC (and higher than the BAC in other parts of the body). Simpson, “Accuracy and Precision of Breath Alcohol Measurements for Subjects in the Absorptive State”, 33(6) Clinical Chemistry 753 (1987).
Gas Chromatography in Breath Testing:
Gas-liquid chromatography, used in the outdated Intoximeter 3000, is a form of breath testing rarely encountered today. The process works by using photocells to analyze the color change of a redox (oxidation-reduction) reaction. A breath sample is bubbled through an aqueous solution of sulfuric acid, potassium dichromate, and silver nitrate. The silver nitrate acts as a catalyst, allowing the alcohol to be oxidized at an appreciable rate. The requisite acidic condition needed for the reaction might also be provided by the sulfuric acid. In solution, ethanol reacts with the potassium dichromate, reducing the dichromate ion to the chromium (III) ion. This reduction results in a change of the solution’s color from red-orange to green. The reacted solution is compared to a vial of non-reacted solution by a photocell, which creates an electric current proportional to the degree of the color change; this current moves the needle that indicates BAC.
Like other methods, breath testing devices using chemical analysis are somewhat prone to false readings. Compounds which have compositions similar to ethanol, for example, could also act as reducing agents, creating the necessary color change to indicate increased BAC.
Myths:
A common myth is that breath testers can be “fooled” (that is, made to generate estimates making one’s blood alcohol content appear lower) by using certain substances. An episode of the Discovery Channel’s MythBusters tested substances usually recommended in this practice — including breath mints, mouthwash, and onion — and found them to be ineffective. Adding an odor to mask the smell of alcohol might fool a person but it does not change the actual alcohol concentration in the body or on the breath. Interestingly, substances that might actually reduce the BAC reading were not tested on the show: a bag of activated charcoal concealed in the mouth would absorb alcohol vapor; an oxidizing gas (such as N2O, Cl2, O3 etc.) would fool a fuel cell type detector; or an organic interferent would fool an infra-red absorption detector. The infra-red absorption detector is especially vulnerable to countermeasures because it only makes measurements at particular discrete wavelengths rather than producing a continuous absorption spectrum.
On the other hand, products such as mouthwash or breath spray can “fool” breath machines by significantly raising test results. Listerine, for example, contains 27% alcohol. A breath machine makes a reading from alcohol in the blood diffusing into the lung rather than directly from the mouth, and it applies a “partition ratio” of 2100:1 in computing blood alcohol concentration. Using Listerine confuses this process, resulting in a false high test reading.
This was clearly illustrated in a study conducted with Listerine mouthwash on a breath machine and reported in an article entitled “Field Sobriety Testing: Intoxilyzers and Listerine Antiseptic”, published in the July 1985 issue of The Police Chief (page 70). [10] Seven individuals were tested at a police station, with readings of .00%. Each then rinsed his mouth with 20 milliliters of Listerine mouthwash for 30 seconds in accordance with directions on the label. All seven were then tested on the machine at intervals of one, three, five and ten minutes.
The results indicated an average reading of .43% blood-alcohol concentration — indicating a level that, if accurate, approaches lethal proportions. After three minutes, the average level was still .20%, despite the absence of any alcohol in the system. Even after five minutes, the average level was .11%, which is well over the legal limit.
In another study, reported in 8(22) Drinking/Driving Law Letter 1, a scientist tested the effects of Binaca breath spray on an Intoxilyzer 5000. He performed 23 tests with subjects who sprayed their throats and obtained readings as high as .81% — far beyond lethal levels. The scientist also noted that the effects of the spray did not fall below detectable levels until after 18 minutes.